How Human Tragedies Led to GMP and SOPs
In today’s world, it’s unthinkable not to have GMP and SOPs if your organization is engaged in activities that affect human health. But where did it all begin? Why so much emphasis on SOPs in the regulations by FDA, EU and WHO? To answer this question, taking a quick glance at the tragic history of GMP (Good Manufacturing Practice) would help.
Sulfanilamide disaster
Let’s start in 1937 when the Sulfanilamide disaster struck the US. Until then, Sulfanilamide, a drug for treating streptococcal infections, was not available in liquid form. But in response to the market demand, the manufacturer decided to produce it in liquid form, for which they used diethylene glycol as a solvent. Before sending Elixir Sulfanilamide, a liquid drug, into the market, it was tested for flavor, appearance and fragrance but not for toxicity. Unfortunately, the drug turned out to be highly toxic because of diethylene glycol and killed more than 100 patients in the United States in 1937.
The public outcry caused by the tragic incident led to the passing of the 1938 Federal Food, Drug, and Cosmetic Act.

Thalidomide trajedy
A similar tragedy struck Europe in 1960. Thalidomide was originally prescribed as a tranquilizer for countering sleeplessness in the post-war US and Europe. But the doctors started prescribing it for an off-the-label purpose: alleviating morning sickness in pregnant women. That’s when the unprecedented tragedy struck. As the drug was unsafe for pregnant women, many children born to these women were left with shorter or no limbs.
This tragedy led to the first GMP regulations in the US in 1963.
GMP is born
It was a series of human health tragedies like the Sulganlimide and Thalidomide disasters that progressively led to the Good Manufacturing Practice (GMP) we know it today. In 1978 GMP regulations became a law in the US. Overall, the timeline of human tragedies and GMP is shown below.
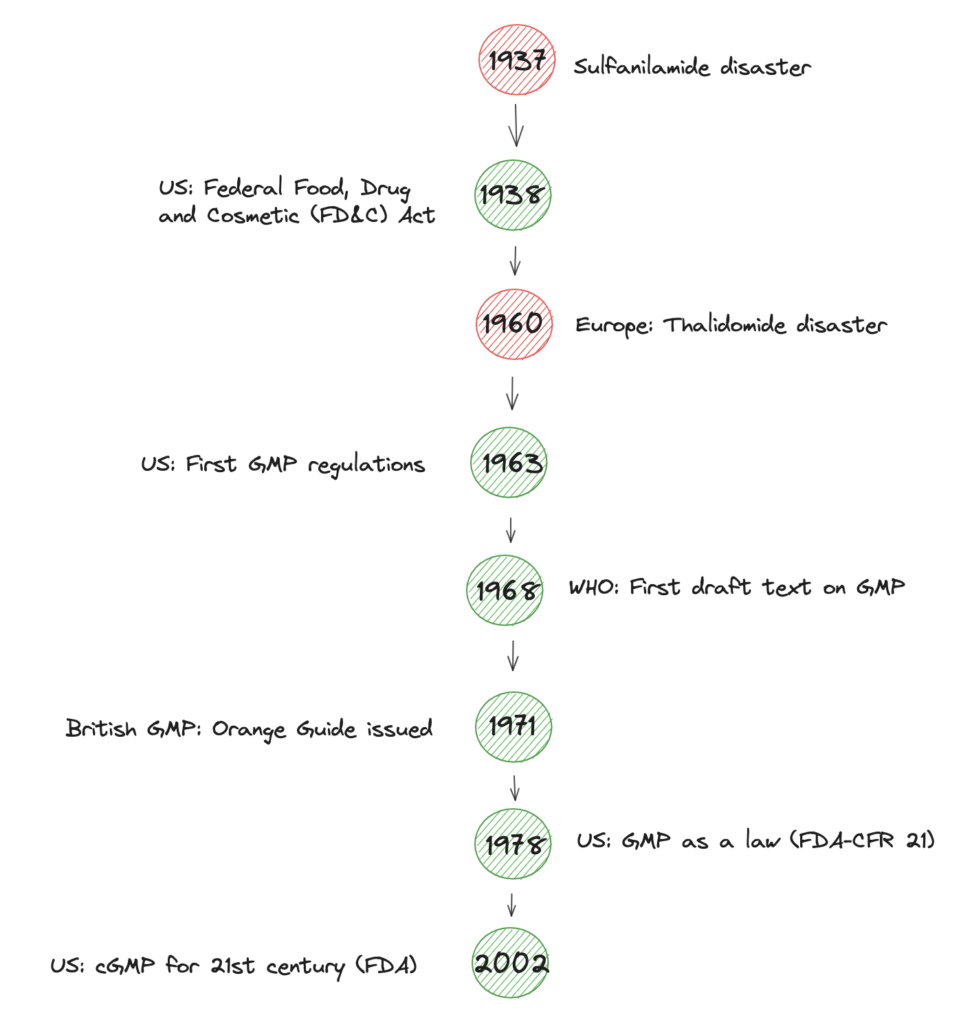
Besides others, one of the core requirements of GMP is SOPs. Basically, organizations must have written procedures for all critical processes and follow them to ensure the quality and safety of their products and services.
If you’re someone who routinely deals with SOPs and can connect the dots between SOPs and GMP–and GMP and its tragic history, you can probably appreciate why the quality of SOPs matters. Some people paid with their lives to lead us to the idea of writing down procedures and following them.
